ScinnoHub Pharmaceuticals Announces China Clinical Trial Approval for BT-114143 Injection
ScinnoHub Pharmaceutical Co., Ltd. ("ScinnoHub") announced that National Medical Products Administration (NMPA) has approved the Investigational New Drug (IND) application of BT-114143 injection for various bleeding-related conditions caused by hyperfibrinolysis. A first-in-human, phase I trial is being planned to begin in the last quarter of 2022. ScinnoHub is planning to apply for a U.S. Food and Drug Administration (FDA) IND application.
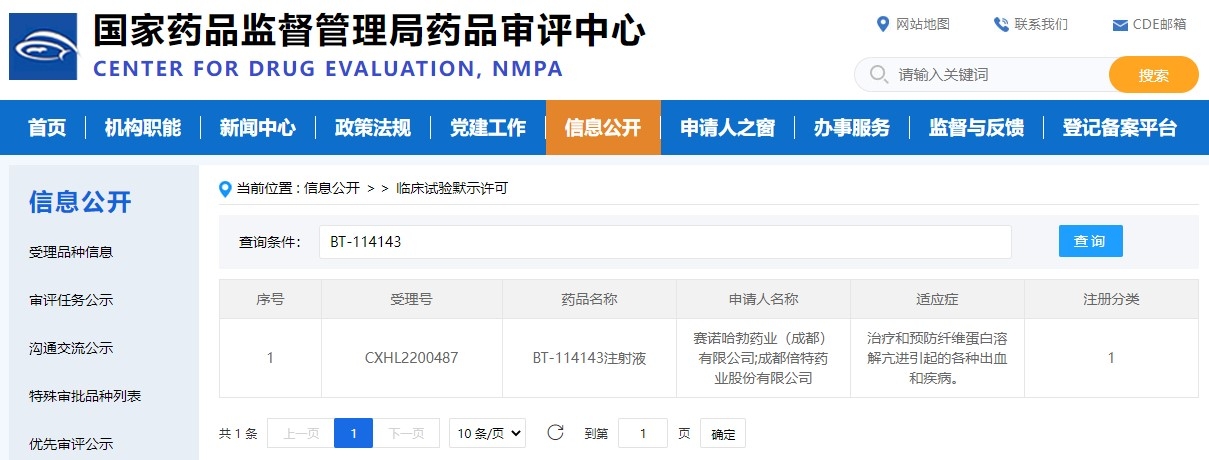
From CDE
Research Status of Antifibrinolytic Drugs
Antifibrinolytic drugs inhibit the conversion from plasminogen to plasmin and subsequent degradation of fibrin, thereby exerting its hemostatic effect. Its development goes back to the 1940s and 1950s.
Epsilon-aminocaproic acid (EACA), a synthetic derivative of the lysine which had an inhibitory effect on plasminogen, was developed in 1952 by Utako Okamoto and Shosuke Okamoto research group. EACA has been widely used clinically since then, but large doses were required along with gastrointestinal (GI) side effects such as nausea. Therefore, a series of antifibrinolytic compounds were developed in 1960s, including 1-(aminomethyl)-cyclohexane-4-carboxylic acid (AMCHA), 4-aminomethylbenzoic acid (PAMBA). In 1964, Utako Okamoto and Shosuke Okamoto research group further developed a trans-form isomer of AMCHA, namely tranexamic acid (TXA), which has been extensively used in postpartum hemorrhage, menorrhagia, trauma-associated hemorrhage, and surgical bleeding.
In the last 60 years, there was no development of antifibrinolytic drug except AstraZeneca’s preclinical program of AZD6564.
About BT-114143 Injection
BT-114143 is a novel anti-fibrinolytic small-molecule compound developed by ScinnoHub. This drug is developed for intravenous administration to treat and prevent various bleeding-related conditions caused by hyperfibrinolysis.
BT-114143 exhibited higher binding affinity for plasminogen than TXA in vitro. Moreover, BT-114143 was 10 times as potent as TXA in inhibiting human whole blood fibrinolysis ex vivo.
Based upon its superior potency, ScinnoHub is developing BT-114143 other formulation, which is intended for various trauma conditions, such as natural disasters, traumatic accidents and battlefield emergencies.
About ScinnoHub
ScinnoHub, a pharmaceutical company based in Chengdu, China, focuses on the development of innovative small-molecule drugs and precision therapeutic platforms with extensive pipelines covering cancer, autoimmune diseases, rare diseases, as well as targeted radioligand therapy and RNA splicing technologies. We strive to address unmet medical needs and develop innovative treatments that will benefit patients
For more information, please see the Company’s website at:http://www.scinnohub.com/en/
Forward-Looking Statements
This press release contains forward-looking statements regarding future plans, expectations and outlooks, including but not limited to the possibility of anticipated clinical development, regulatory milestones and commercialization of BT-114143. These statements are inherently subject to certain risks and uncertainties. When using words such as "intend," "likely," "estimate," "target," "potential," "anticipate," "believe," "predict," "expect," "intend," and other similar words , which relate to ScinnoHub, are intended to identify forward-looking statements. All forward-looking statements are based on information currently available to ScinnoHub, and ScinnoHub undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.
References
[1] Okamoto S. Plasmin and antiplasmin [J]. The Keio Journal of Medicine, 1959, 8(4):211-7.
[2] Tengborn L, Blombäck M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival [J]. Thrombosis research, 2015, 135(2): 231-42.
[3] Okamoto S, Okamoto U. Aminomethylcyclohexanecarboxylic acid: AMCHA, a new potent inhibitor of fibrinolysis [J]. Keio J. Med. 1962, 11, 105-15.
[4] Steinmetzer T, Pilgram O, Wenzel B M, et al. Fibrinolysis Inhibitors: Potential Drugs for the Treatment and Prevention of Bleeding [J]. J Med Chem, 2020, 63(4): 1445-72.
[5] Okamoto S, Sato S, Takada Y, et al. An active stereoisomer (trans-form) of AMCHA and its antifibrinolytic (antiplasminic) action in vitro and in vivo [J]. Keio J Med, 1964, 13: 177-85.
[6] Cai J, Ribkoff J, Olson S, et al. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients [J]. European journal of haematology, 2020, 104(2): 79-87.






